Sulfides
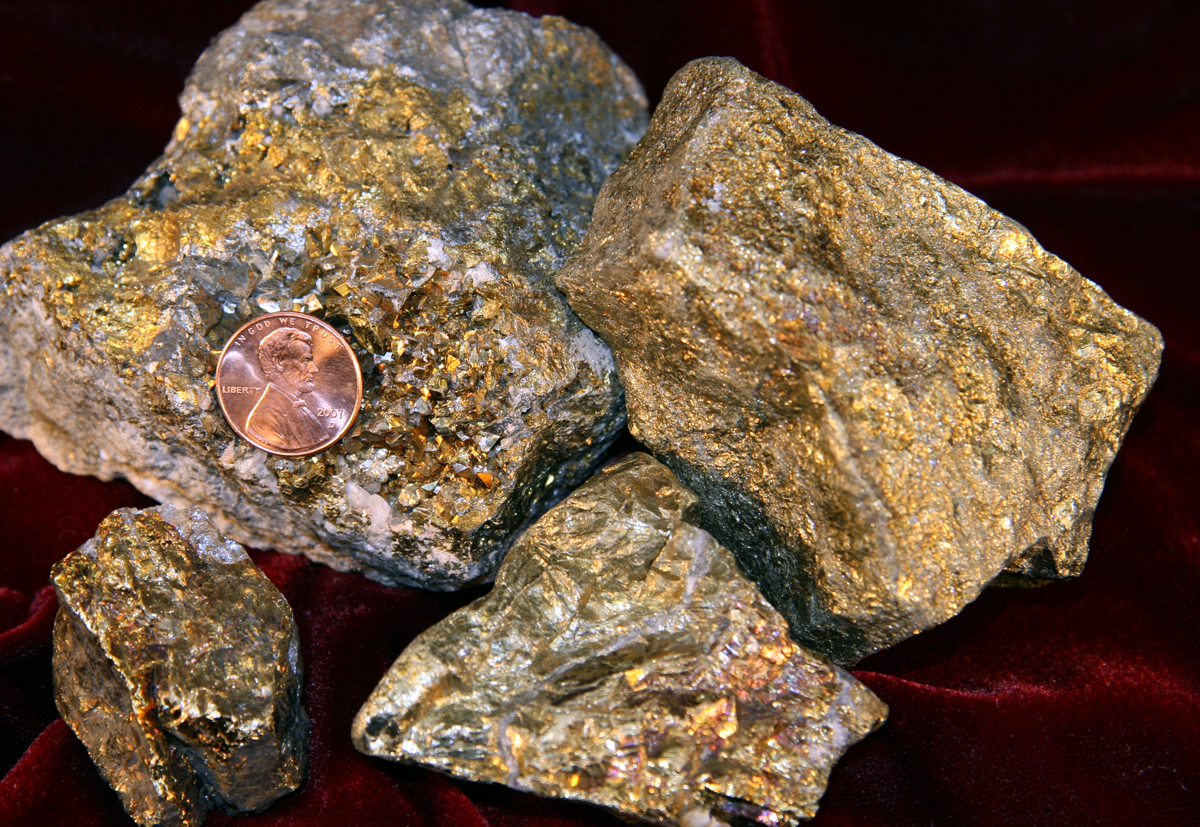
Chalcopyrite properties
| Chemical Composition | CuFeS2 – Copper Iron Sulfide |
| Color | Brass yellow, often with an iridescent tarnish that may give it a green, blue, or purple cast. |
| Cleavage | Cleavage is very poor. |
| Hardness | 3.5 – 4, brittle nature |
| Specific Gravity | 4.1-4.3 (average for metallic minerals) |
| Luster | Metallic, opaque |
| Streak | Greenish black to black |
Did you know...
Chalcopyrite was the mineral upon which Bronze Age civilizations were built. Within the last century, it also became the mineral foundation for our modern electrical age. Our primary source of copper, chalcopyrite’s name comes from the Greek words ‘chalkos’ and ‘pyrites’, which respectively mean ‘copper’ and ‘striking fire’. With its metallic luster and bright golden color, chalcopyrite can fool people into thinking it is gold. It is one of two minerals, the other being pyrite, that are commonly known as ‘fool’s gold’.
Description and Identifying Characteristics
Chalcopyrite is a striking, bright yellow, metallic mineral that occurs in nearly all sulfide deposits. Initially, it may be easy to confuse chalcopyrite, or copper pyrite (CuFeS2), with pyrite (FeS2), but the two can be distinguished by their relative hardness and chalcopyrite’s iridescent tarnish. Pyrite is the harder of the two and cannot be easily scratched by a nail, unlike chalcopyrite. In its massive variety, chalcopyrite is one of the minerals most often mistaken for gold. While gold is ductile and malleable, chalcopyrite is brittle and will shatter if struck.
In Our Earth: The Geologic Importance of Chalcopyrite
Chalcopyrite is easily the most widespread copper-bearing mineral. A common mineral found in almost all sulfide deposits, chalcopyrite usually occurs in medium-temperature and high-temperature hydrothermal veins in igneous rocks or metamorphosed igneous rocks. Some economic chalcopyrite deposits form as hydrothermal fluids dissolve copper from igneous rocks and then precipitate it in surrounding contact-metamorphosed sedimentary rocks. Chalcopyrite is most often found with pyrite and other sulfide minerals, as well as sphalerite, galena, dolomite, tourmaline or quartz. It can oxidize to form a number of minerals such as malachite, azurite, and cuprite.
In Our Society: The Economic Importance of Chalcopyrite
Throughout human history, chalcopyrite has been our leading source of copper. This is despite having a relatively low copper yield (only 25% of its atoms are copper) compared to other copper minerals such as chalcocite and cuprite (both with 67% yields), or bornite and covellite (that have 50% yields). However, chalcopyrite is much more abundant than the other copper-bearing minerals and is far more widely distributed so it remains our most important copper source.
In many societies copper was the first known metal to be widely worked, and for over six thousand years copper mining has remained a crucial industry. Copper is easily worked and can be mixed with zinc to make brass, or with tin to make bronze. Before people learned to smelt iron, bronze was the most durable, widely worked and economically most important metal. From its earliest civilizations to the Roman era, political and military strength in the Mediterranean revolved around the control of vast copper and other metal deposits in uplifted ancient seafloor rock. Some Spanish chalcopyrite and pyrite bodies have been continuously mined for over three thousand years to produce copper and sulfur, along with other associated minerals such as gold.
More recently, copper’s high conductivity, softness, and resistance to corrosion, have given it a critical role in generating and distributing electrical power. Copper is easily worked and relatively cheap so it is used for the bulk of the wiring that connects our society’s electrical systems. Although this is currently its most important contribution, copper has also been in demand for uses ranging from coinage to building decorations. Medical uses for chalcopyrite range from ancient healing methods to modern acupuncture.
Chalcopyrite in the Upper Midwest:
Chalcopyrite occurs as massive sulfide vein deposits in the middle and late Precambrian volcanic rocks of northern Wisconsin and northern Minnesota along the Canadian border. In these veins, massive chalcopyrite is interwoven with pyrite and sphalerite. The lower part of the Duluth Complex southwest of the Boundary Waters Canoe Area holds particularly rich deposits of chalcopyrite and nickel sulfides. When first mapped out in 1980, these deposits held nearly a fourth of the United States’ known copper reserves and over a tenth of the world’s known nickel supply. Chalcopyrite also occurs in lesser quantities as vein deposits in igneous and metamorphic rocks across the region.
Southeastern Minnesota and southwestern Wisconsin comprise the northern extent of the Upper Mississippi Valley lead-zinc mining district. Throughout this region, chalcopyrite occurs in hydrothermal veins running through the area’s Paleozoic sedimentary rocks. Within these veins, chalcopyrite occurs as massive deposits or as crystals interwoven with crystals of galena, sphalerite, and barite.
Native copper found along the southern shore of Lake Superior is perhaps the best regional testimony to the longstanding economic impact of copper and chalcopyrite. Copper ingots traded as jewelry by Indigenous nations were the driving force behind the first European exploration of the Upper Midwest. French explorers originally pushed into the region in search of copper and other metal wealth, only later settling for the fur trade when they failed to find large ore deposits.
Chalcopyrite Gallery
Commonly confused with...
Chalcopyrite’s bright yellow color and brilliant metallic luster distinguish it from most common minerals. Pyrite and gold are the only natural materials that are easily confused with chalcopyrite and the three can be distinguished by their hardness and response to stress.
Pyrite:
At first glance, pyrite and chalcopyrite are similar in their color and shiny metallic luster. However, the two can be distinguished by their hardness. Chalcopyrite is a soft mineral that can be scratched by a nail, while pyrite is hard enough that a nail cannot dig into its surface. Unlike pyrite, chalcopyrite also weathers to display an iridescent tarnish.
The two minerals also usually occur in different forms. Pyrite is most often found as crystal masses that exhibit obvious flat planes and cubic shapes. Although chalcopyrite can occur as crystals, it most often occurs as masses that lack flat planes or obvious geometric shapes.
Gold:
Gold is a highly valued mineral that may initially be confused with massive chalcopyrite. Indeed, chalcopyrite is often referred to as ‘Fool’s Gold’. The two may be distinguished by chalcopyrite’s color and brittle nature. Chalcopyrite has a brassy yellow color that is distinct from gold’s more buttery yellow. Gold is also very ductile and easily deformed by pressure, whereas chalcopyrite is brittle and will shatter if struck. Finally, most specimens of chalcopyrite exhibit a slight, to a pronounced, iridescent tarnish that does not occur in gold.